Boltzmann Constant and Avogadro's Number
Avogadros law Opens a modal The van der Waals equation Opens a modal. Electron mass energy J m e c 2.

Mathtype The Gas Constant R Is Defined As The Avogadro Constant Na Multiplied By The Boltzmann Constant K It Is Mostly Known For Appearing In The Ideal Gas Law And Physically
The pressurevolume product rather than energy per temperature increment per particle.

. When we use gmol we describe the weight of one mole of a given substance. Boltzmann distribution Boltzmanns constant bond chemical boomerang Bose-Einstein condensation Bose-Einstein statistics bosons. The relationship between the gas constant R and the Boltzmann constant k B.
The ideal gas constant is the combination of Boyles law Avogadros number Charless law and Gay-Lussacs law. Avogadros number P pressure default is 1 atmosphere R gas constant entropy due to electronic motion. The molar gas constant also known as the gas constant universal gas constant or ideal gas constant is denoted by the symbol R or RIt is the molar equivalent to the Boltzmann constant expressed in units of energy per temperature increment per amount of substance ie.
Gravitation constant G 66710 11 m 3 kg 1 s 2. 8314 J mol-1 K-1. The molar gas.
Because of the relatively recent definition change use care when comparing calculations prior to 2019 because the values for R are slightly different. Kinetic theory of Gases. The ideal gas constant and the Boltzmann constant k B are related by Avogadros constant N AThe.
Although their values are identical they describe. 138 x 10-23 J K-1. Coulomb constant 14πε 0 8987551792314 10 9 N m 2.
The average speed is the sum of the speeds of all the molecules divided by the number of molecules. Avogadros number is approximately 6022140857741023 mol1. Or 1380649 1016 erg per kelvin.
Where c p is the specific gas constant at constant pressure and c v is the specific heat capacity at constant volume. The constant α depends on the. Electron charge to mass ratio-em e-17588 x 10 11 C kg-1.
The specific gas constant is very useful in engineering applications of thermodynamics. Thus the gas constant R can be given as Gas constant R 8314459848 Jmol 1 K 1. Kinetic molecular theory of gases.
Molar gas constant R 8314 Jmol K Avogadros number NA 6023 10 23 mol 1. Learn About Using Constants in Java. Charge of electron e 1602 10 19 C.
008206 L atm mol-1 K-1. The gas constant corresponds to the work performed by one mole of an ideal gas when heated by 1 K at constant pressure. Rk B N A.
The name Avogadros number was coined in 1909 by the physicist Jean Perrin who defined it as the. The relationship between the Faraday constant F and the elementary charge e. 2818 x 10-15 m.
Molar mass is often confused with atomic or molecular mass. Permittivity of vacuum 0 885 10 12 Fm. Boltzmanns entropy equation which incorporates Boltzmanns.
Where k is the Boltzmann constant with value 198 X 10²³ JK and T is the Temperature. Boltzmanns Constant and Ideal Gas Constant. The universal gas constant R is defined as the product of Avogadro constant N A number of particles in one mole of gas and Boltzmanns constant k.
The mole and Avogadros number Opens a. Avogadros number is defined to be the number of atoms in 12 grams of carbon and is approximately six followed by 23 zeroes. Boltzmanns constant Opens a modal Heat capacity at constant volume and pressure Opens a modal Kinetic molecular theory of gases.
The digits inside the parentheses are the uncertainty in the measurement of the gas constant value. A mole is a unit used for measuring matter. Its dimension is work per amount per temperature and the constant is exactly defined as 831446261815324 JK¹mol¹.
Avogadros number background radiation 3K bag model quarks ballistic pendulum. The Avogadro constant is named after the Italian scientist Amedeo Avogadro 17761856 who in 1811 first proposed that the volume of a gas at a given pressure and temperature is proportional to the number of atoms or molecules regardless of the nature of the gas. Boltzmann constant the energy of the n-th energy level the degeneracy of the n-th energy level symmetry number for rotation h Plancks constant m.
831446261815324 JK 1 mol 1. 1602 10-19 C. Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules.
One mol contains exactly 60221407610²³ elementary particles this number is called the Avogadros Number. The Foundation for Successfully Teaching. 8187 x 10-14 J.
The gas constant is also defined as the Avogadro constant N A multiplied by the Boltzmann constant k. Both Avogadros number and the Boltzmann constant were given exact numerical values. The physical significance of k is that it Boltzmann constant symbol k a fundamental constant of physics occurring in nearly every statistical formulation of both classical and quantum physics.
Boltzmann constant k 138 10 23 JK. The root-mean-square speed is the square root of the average speed-squared. The molar gas constant R is defined as Avogadros number times the Boltzmann constant.
Faradays Constant F Ne. As a consequence the gas constant also now has an exact value. 9649 10 4 C mol electrons-1.
6022 10 23 mol-1. Boltzmanns Constant k b 1381 10-23 J K-1. Gas Constant In Different.
The number of units in one mole of any substance is called Avogadros number or Avogadros constant. Avogadros Number Example Chemistry Problem. Ideal Gas Constant R Nk b.

A Mind Dump Of Mathematics Deriving Boltzmann S Constant

Boltzmann Constant Definition And Units

Boltzmann S Constant And Avogadro S Number Physics Lecture Sabaq Pk Youtube
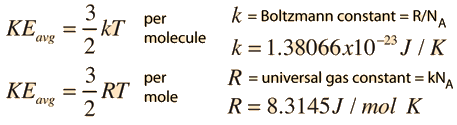
No comments for "Boltzmann Constant and Avogadro's Number"
Post a Comment